By Shaldine Sutton
The Western Cape Blood Service (WCBS) is licenced to manufacture and sell reagents to pathology laboratories and has been doing so for more than 20 years. This licence permits manufacture in terms of section 22C (1)(b) of the Medicines and Related Substances Act. Our laboratory, with only five employees, operates within WCBS’s Processing Department in Cape Town.
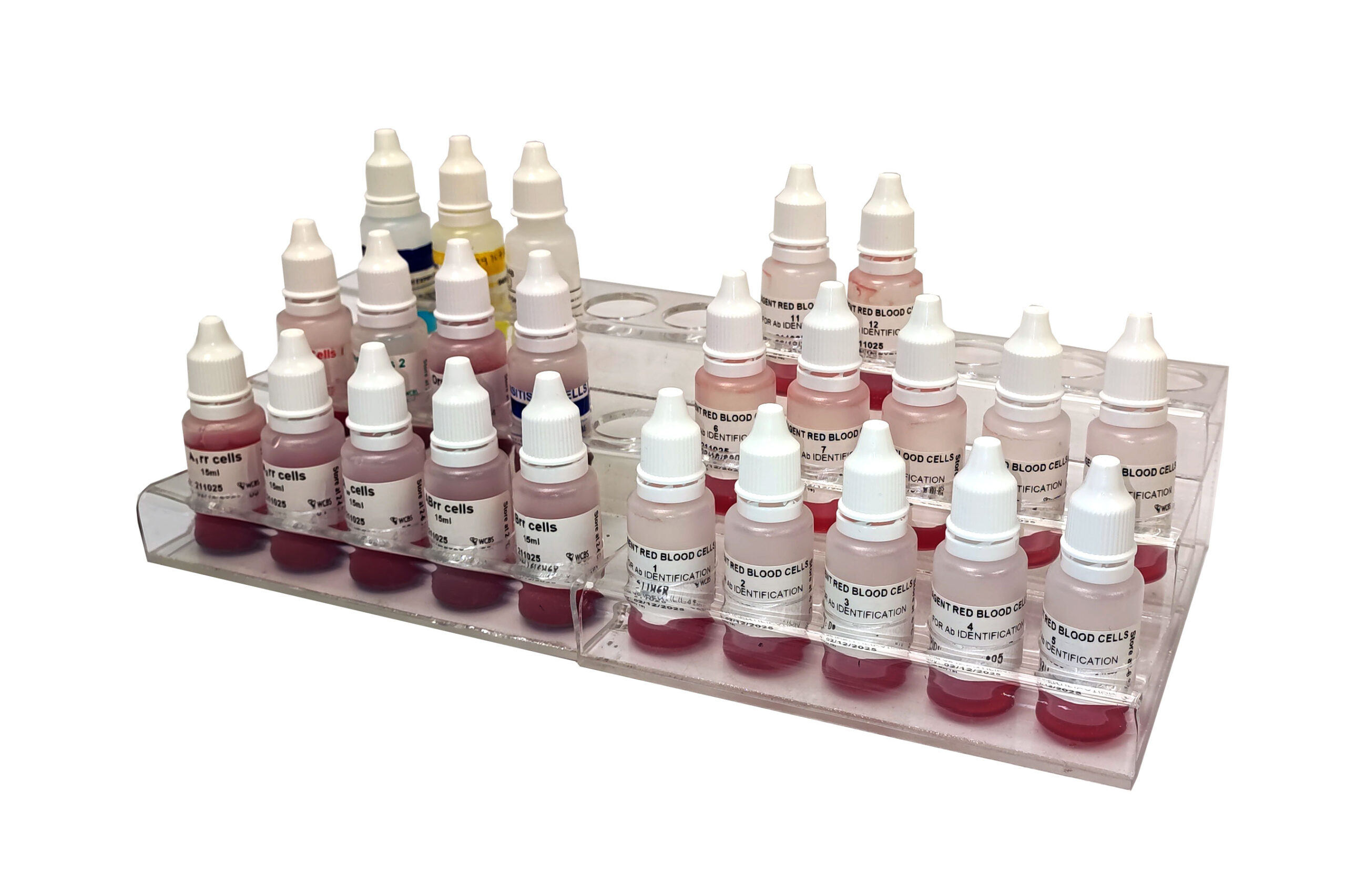
The Reagents Laboratory supplies approximately 20 pathology laboratories, both private and state, of which three are beyond South Africa’s borders. The reagents are for blood grouping, irregular red cell antibody screening and identification. These reagents cause agglutination when the cells express the specific red cell antigen. The benefits of red cell blood grouping, irregular antibody screening, and identification are to prevent serious transfusion reactions and to detect haemolytic disease of the newborn, thereby enabling safer blood management in transfusion and obstetrics.
The South African Health Products Regulatory Authority (SAHPRA) implemented a requirement that all companies that require In Vitro Medical Device (IVD) licensing must be ISO 13485 certified. This aligns with international standards, enhances quality and safety, and ensures a commitment to high-quality health care products, ensuring medical devices are safe and perform as intended.
WCBS laboratories are ISO 15189 accredited, which is an international standard specifying medical laboratory quality and competence, with a formalised Quality Management System in place. In order to retain our SAHPRA license WCBS was required to obtain ISO 13485 certification, which was successfully achieved in May 2025.
The reagent ordering process is available on the WCBS website, where the Marketing Department will provide a quotation. Individual bottles of 15 ml of blood grouping antisera, namely, Anti-A, Anti-B, Anti-AB and blood grouping and irregular antibody screening cells, namely, A1rr, A2rr, Brr, Orr, Sensitised Cells, Screen Cells 1, Screen Cells 2 are available. Due to red cell expiry, production is done in four weekly cycles. Manufacture and supply are according to customer requirements, with delivery services available, if required.
For more information about WCBS blood reagents, contact Shaldine Sutton, Head — Processing, Reagents and Inventory Control (Shaldine@wcbs.org.za).





